Categories | News & Politics
Article
Viona Pharmaceuticals Announces Recall for Type 2 Diabetes Drug Over Cancer Concerns
December 31st, 2021
•
News & Politics
•
2 minute read
Viona Pharmaceuticals Announces Recall for Type 2 Diabetes Drug Over Cancer Concerns
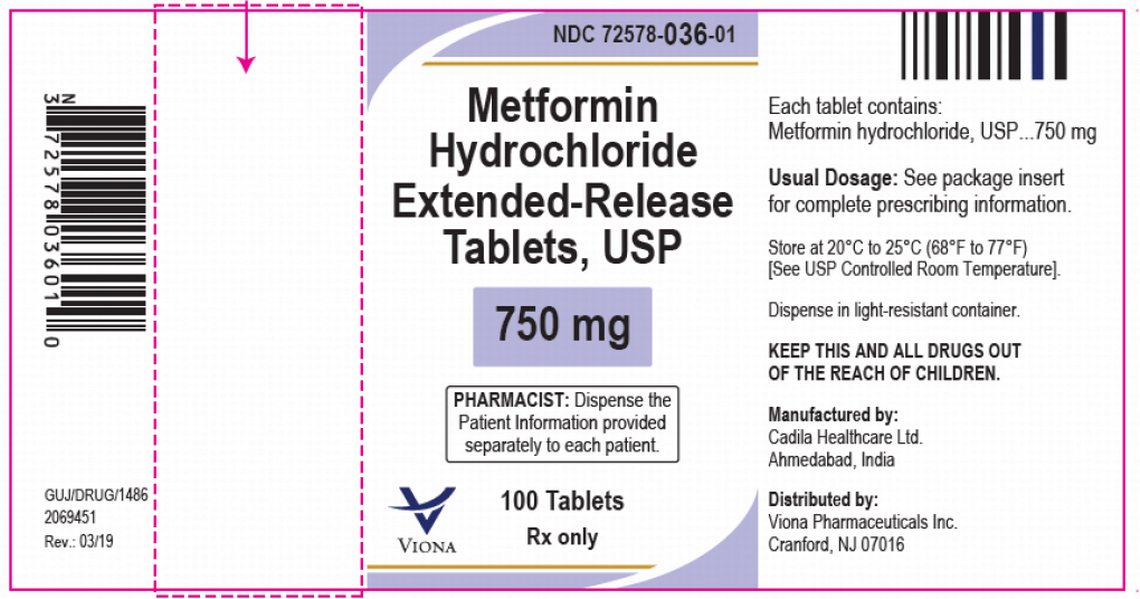
Viona Pharmaceuticals is recalling certain lots of type 2 diabetes drug 750 mg strength metformin over concerns that the drug contains too much NDMA. NDMA, otherwise known as N-nitrosodimethylamine, is a probable human carcinogen and was linked to countless metformin recalls in 2020.
 FDA Building 21 stands behind the sign at the campus's main entrance and houses the Center for Drug Evaluation and Research; image by FDA, Public domain.
FDA Building 21 stands behind the sign at the campus's main entrance and houses the Center for Drug Evaluation and Research; image by FDA, Public domain.
M008130-133, exp. date 06/2022
M010080-81, exp. 07/2022
M011029-32, exp. 08/2022
M013394-96, M013966-67, exp. 09/2022
M100831-32, exp. 12/2022
M100833-34, M101267, M102718-20, exp. 01/2023
M102721-22, M104172-76, exp. 02/2023
M105889-90, exp. 03/2023
Sources:
Company recalls a diabetes drug because it might have too much of a carcinogenFDA: Diabetes Medication Recall Due To Cancer Concerns, What Is Metformin?
About Brianna Smith
Brianna Smith is a freelance writer and editor in Southwest Michigan. A graduate of Grand Valley State University, Brianna has a passion for politics, social issues, education, science, and more. When she’s not writing, she enjoys the simple life with her husband, daughter, and son.
